Introduction
Trangia alcohol stoves are very popular here in Australia, according to a recent survey on aus.bushwalking newsgroup users, 27% of bushwalkers are using Trangia stoves(1).
Methylated Spirits is the main alcohol fuel of choice, it is of a very high quality, with a standard of 95-96% ethanol content, but it can leave a residue or sometimes soot on the bottom of cooking pots, adding water to Methylated Spirits is common practice to try and reduce the amount of soot. Trangia instructions suggest ‘To avoid sooting, dilute the fuel with 10% or max. 15% water’ but Trangia do not mention if adding water has any affect on fuel efficiency.
The aim of this study is to investigate if adding water to Methylated Spirits has an effect on the efficiency of Methylated Spirits as a Bushwalking stove fuel?
TESTING
Methylated Spirits/Water mixes from 100% methylated Spirits to 75% methylated Spirits 25%water content by volume where tested in 5% increments, I did try higher water content but I found that the higher water mixes went out very easily and took more time to boil than was practical for field use.
I have access to some very accurate density measuring equipment, repeatability 1E-6 g/cm2 at 20ºC +-0.001ºC, which I used to determine the densities of the Methylated/Water mixes both before the tests and after the tests.
To keep this report short and to the point, I have left out some of the boring technical details, these details will be published if full detail on my soon to be live web site or if someone wants some more detail I can send it to them.
Equipment and testing method
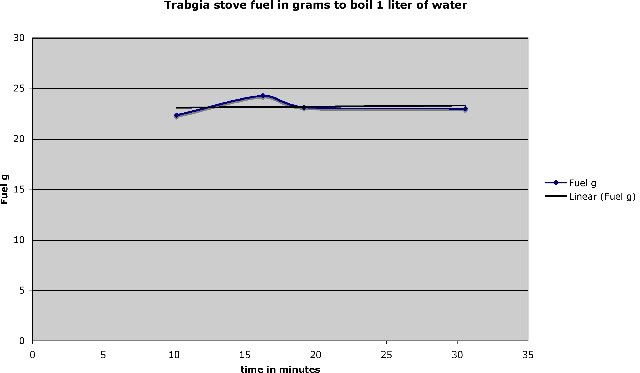
Stove used: Trangia No 27-1, 23-hole burner.
The heating tests where done using some specially built temperature measuring equipment and the data logged on a computer using a program written in Labview, with a National Instruments USB-6008 A-D device. A standard 0.5 liters of water was used in the tests at tap temperature water which was usually around 17ºC, the weight of fuel was measured at start and when the water temperature reached 95ºC the stove was extinguished and the fuel re-measured, the fuel measurement was then recalculated to normalize the results to g fuel used/80ºC. After the stove was extinguished the remaining fuel was weighed and a sample of the remaining fuel mixes was taken
for density measurements. These tests where repeated three times for each fuel mixture and the results where averaged.
The tests were done in my garage elevation 600m, ambient temperature of between 20ºC-25ºC

Testing equipment
Test results charts

Graph 2 Comparing pre-boil densities to post-boil densities
Does the alcohol burning off by itself leaving mostly water
To test this I took a sample of the remaining fuel after the burner was extinguished and then re-measured the density using the density meter. As can be seen from graph below the alcohol/water ratio is very close to being maintained, there was a little more alcohol burned than water evaporated but basically there is very little change in density from pre- to post test density. Why? I am not sure; this will need some more investigating.

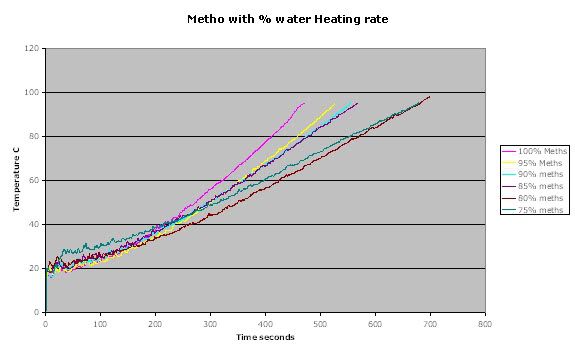
Heating rate
As can be seen from the heating rate the more water added the slower the heating rate.
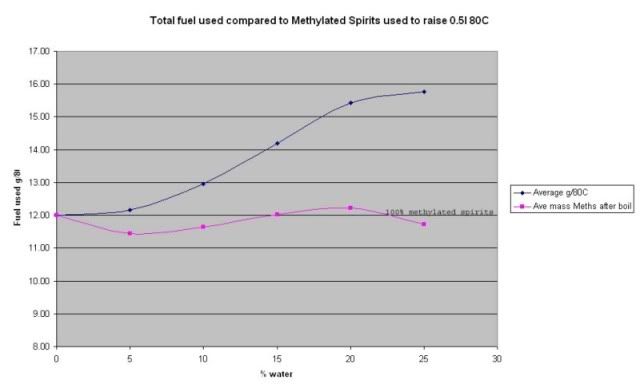
Fuel used to heat 500 ml of water 80ºC (blue line) and Methylated Spirit content in that fuel (pink line).
In the results graph above the blue line, which shows the test results of the total Methylated Spirits/Water mix used to raise the water 80ºC per %, mix. The pink line is the calculated amount of Methylated Spirits used per % mix. Note that the Methylated Spirits results line closely follows the 12.0 grams line which is the 100% Methylated Spirits result.
Conclusion
The aim of this study that was to investigate “if adding water to Methylated Spirits has an effect on the efficiency of Methylated Spirits as a Bushwalking stove fuel?” The answer that I concluded from the tests results is adding up to 25% water concentration by volume to Methylated Spirits does not reduce the efficiency; there is some evidence that in some concentrations 5%-10% it may slightly enhance the efficiency. As to why at the moment I am unsure but I am looking into it.
Is adding Water to Methylated Spirits worth doing, In my opinion NO, the maximum measured improvement to fuel saved is 6% at 10% water added, to get the concentrations right you would need some reasonably accurate measuring equipment and this extra weight and effort would negate some of the advantage. Tests have shown that a blackened pot surface helps to improve heat absorption (2)(3) (Black body radiator effect).
References
(1) http://adunk.ozehosting.com/trangia.html
(2) http://www.bioenergylists.org/stovesdoc ... Berick.pdf
(3) http://www.aprovecho.org/web-content/pu ... s/pub2.htm